Recently,one of the most distinguished meeting in the field of pancreatology—the APA/JPS/CAP/IAP 2024 Annual Meeting, jointly organized by the American Pancreatic Association (APA), the Japan Pancreas Society (JPS), the Chinese Pancreatic Association (CAP) and the International Association of Pancreatology (IAP), was successfully held in Hawaii, USA. At this congress, Professor Dan Cao’s team from West China Hospital of Sichuan University reported the results of a phase 2 clinical trial of serplulimab in combination with chemotherapy and radiotherapy for the first-line treatment of pancreatic cancer in oral presentations.

HANSIZHUANG (serplulimab), Henlius’ first self-developed innovative Anti-PD-1 mAb, is the world’s first anti-PD-1 mAb for first-line treatment of extensive-stage small cell lung cancer (ES-SCLC). Up to date, it has been approved in China and several Southeast Asian countries, benefiting over 90,000 patients. Furthermore,the marketing applications of the first-line treatment for ES-SCLC is under review by the European Medicines Agency (EMA), which is expected to be approved in 2025. Focusing on lung and gastrointestinal cancer, the synergy of HANSIZHUANG with in-house products of the company and innovative therapies are being actively promoted. The company has initiated more than 10 clinical trials on immuno-oncology combination therapies in a wide variety of indications with more than 4,600 subjects enrolled in China, the U.S., Turkey, Poland, Georgia and other countries and regions.
Gastrointestinal tumors are characterized by high incidence and mortality rates both in China and globally. The main types include colorectal cancer, gastric cancer, liver cancer, esophageal cancer, pancreatic cancer, and gallbladder & bile duct cancer. According to global cancer statistics for 2022, there were approximately 4.90 million new cases and 3.32 million deaths due to gastrointestinal cancers worldwide, accounting for about 25% of all new cancer cases and more than 34% of cancer-related deaths[1]. In terms of gastrointestinal cancer, HANSIZHUANG in combination with chemotherapy for the first-line treatment of patients with unresectable locally advanced/recurrent or metastatic ESCC has been approved by the China National Medical Products Administration (NMPA) in September 2023, providing a new treatment option for patients with ESCC. Meanwhile, HANSIZHUANG has led the way with a phase 3 clinical study on neoadjuvant/adjuvant therapies for gastric cancer, striving to benefit gastric cancer patients from the early line of immunotherapy.
The detailed results of the study released at APA/JPS/CAP/IAP 2024 Annual Meeting are as follows:
Title: Phase II Trial of Gemcitabine plus nab-paclitaxel (GnP) Combined with Serplulimab and SBRT for Metastatic Pancreatic Cancer As the First-line Treatment
Leading PI: Dan Cao, West China Hospital of Sichuan University
Presenter: Ke Cheng, West China Hospital of Sichuan University
Study Design:
Immunotherapy (Serplulimab), chemotherapy (GnP) plus SBRT (ICSBRT) as the first-line treatment for mPDAC. The eligible patients without previous treatment, will be administered gemcitabine 1000mg/m2 and nab-paclitaxel 125mg/m2 intravenously on day 1 and day 8 every 3 weeks. Serplulimab 200mg will be administered intravenously on day 1 every 3 weeks, and SBRT delivering 5 fractions of 6.6 Gy to primary tumor or 3 fractions of 8 Gy to metastatic lesion in cycle 2. The primary endpoint was 6-month progression free survival (PFS) rate. The secondary endpoints included overall survival (OS), PFS, ORR, DCR, and safety outcomes.
Results:
Follow up to October 2024, forty-four patients were enrolled.
Primary endpoint:The 6-months PFS rate calculated through KM curve is 74.48%.
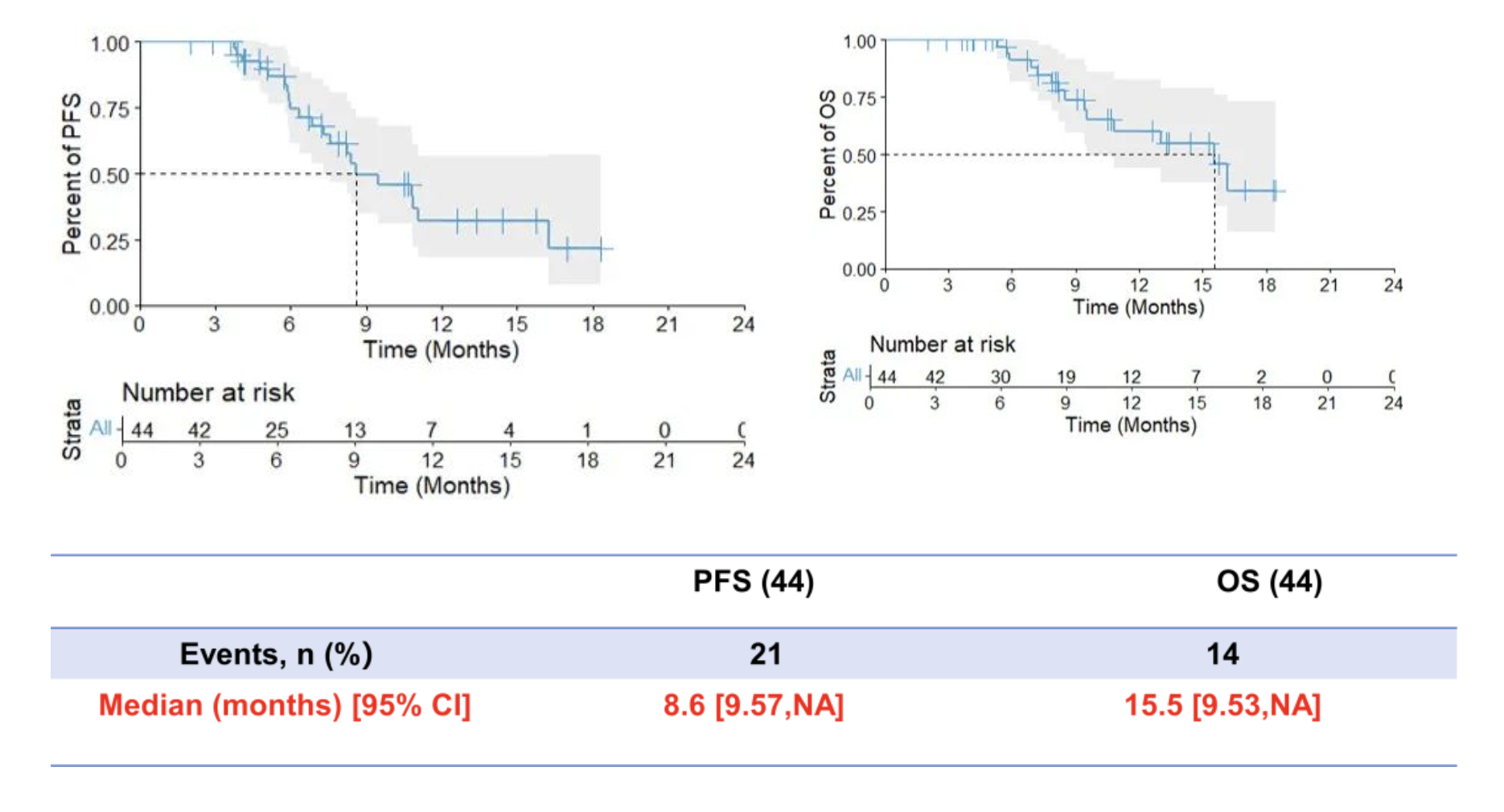
Secondary endpoints: an mPFS of 8.6 months and an mOS of 15.5 months.
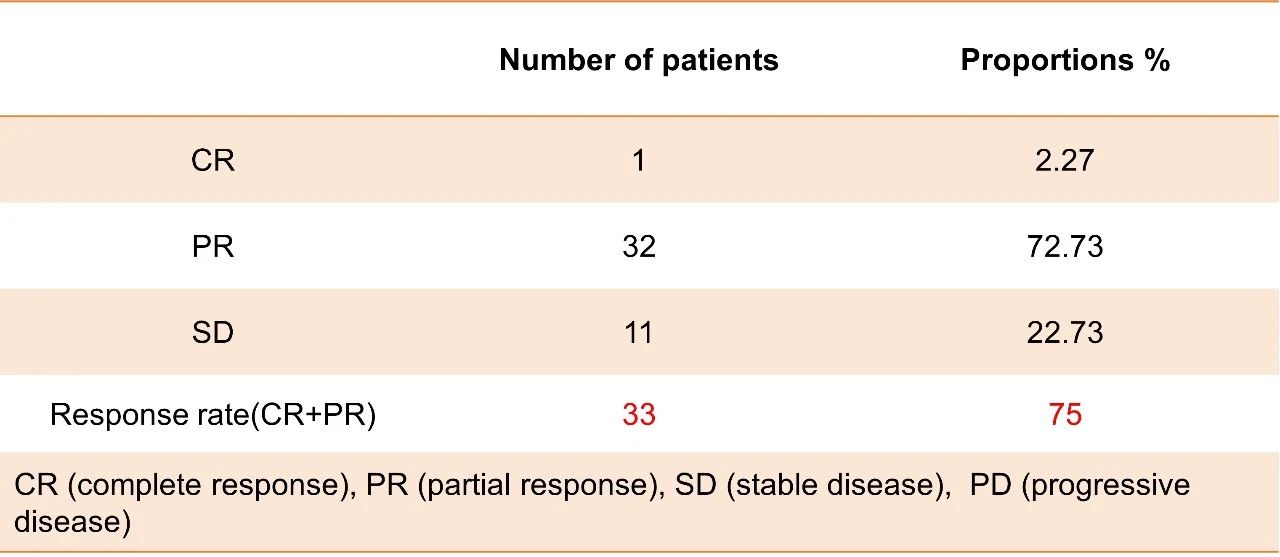
Secondary endpoints: The ORR of the study was 75%, and the DCR was 100%.
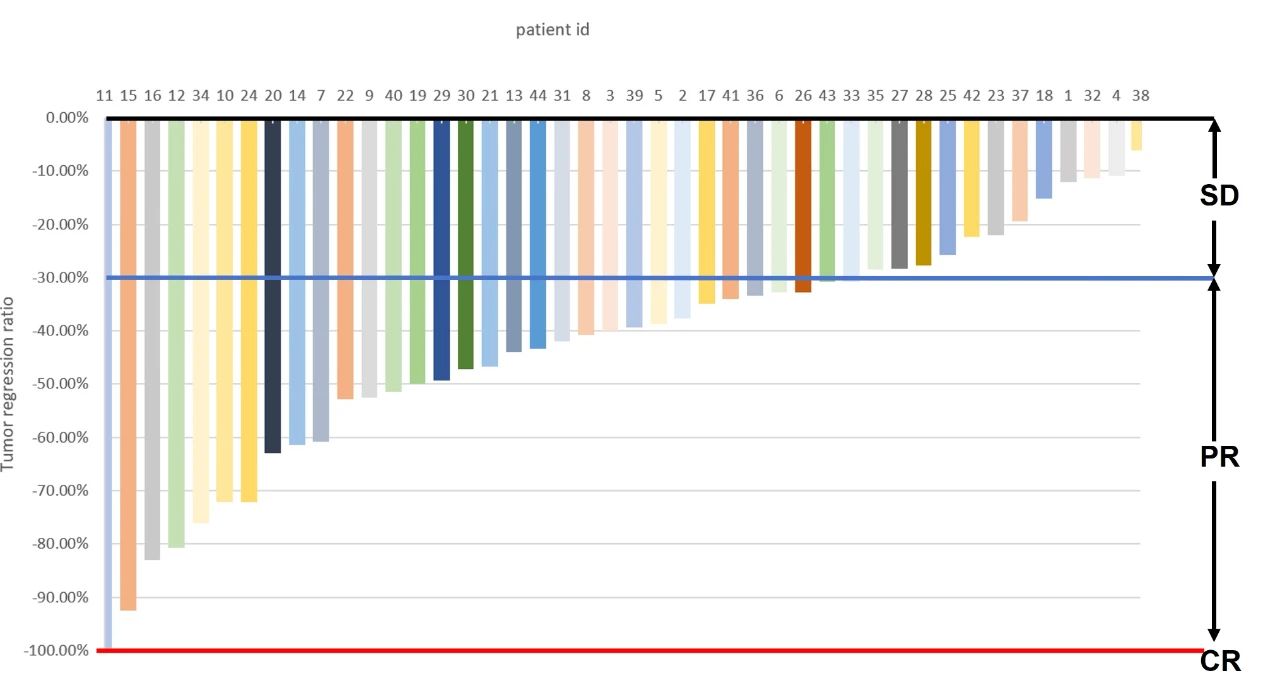
Tumor regression ratio: Waterfall plot of the best percent change in target lesion diameter from baseline, colored according to best of response (n=44). All patients experienced tumor regression in the target lesion, 33 patients achieved PR/CR.
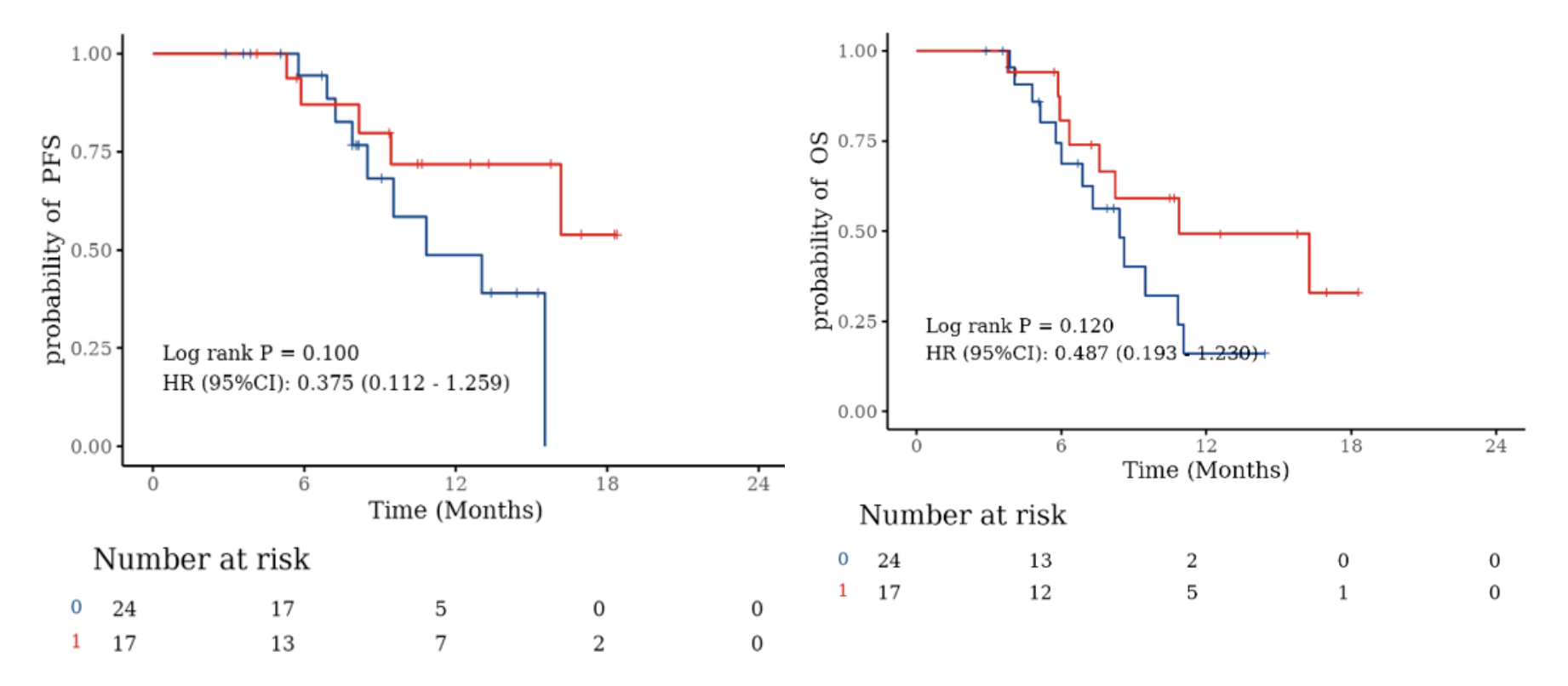
Subgroup survival analysis: according to whether received radical resection before enrollment. No significant difference was observed in PFS and OS.
Safety analysis: the incidence of grade 3 or higher TEAEs was 79.55%, with no serious TEAEs occurring. The frequent TEAEs included anorexia, neutropenia, leukopenia, fatigue, vomiting, rash, and nausea.
Conclusion:
The interim results of this phase II study has met our preset primary endpoint with 74.48% in 6-month PFS rate, achieving ORR with 75%, DCR with 100%, median PFS with 8.6 months, median OS with 15.5 months, and manageable safety profile.
We are the first to report the favorable clinical efficacy and safety of ICIs plus SBRT combined with chemotherapy in first-line mPC. And ICSBRT presented promising efficacy, and this supported ongoing this combination as the first-line treatment in patients with mPDAC.
【Reference】
[1] Bray F, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
