From 11 to 13 December 2024, the 2024 European Society for Medical Oncology Immuno-Oncology (ESMO IO) Congress took place in Geneva, Switzerland, and online. At this congress, the results of a phase 2 clinical trial of serplulimab in combination with chemotherapy in the neoadjuvant treatment of oesophagexal squamous cell carcinoma have been published in poster presentation. This study was led by Professor Ming Wu and and Professor Hong Shen from the Second Affiliated Hospital Zhejiang University School of Medicine.
HANSIZHUANG (serplulimab), Henlius’ first self-developed innovative Anti-PD-1 mAb, is the world’s first anti-PD-1 mAb for first-line treatment of extensive-stage small cell lung cancer (ES-SCLC). Up to date, it has been approved in China and several Southeast Asian countries, benefiting about 90,000 patients. In China, it has been approved by the NMPA for the treatment of 5 indications including squamous non-small cell lung cancer (sqNSCLC), ES-SCLC, esophageal squamous cell carcinoma (ESCC) and non-squamous non-small cell lung cancer (nsNSCLC). Furthermore,the marketing applications of the first-line treatment for ES-SCLC is under review by the European Medicines Agency (EMA), which is expected to be approved in 2025. Focusing on lung and gastrointestinal cancer, the synergy of HANSIZHUANG with in-house products of the company and innovative therapies are being actively promoted. The company has initiated more than 10 clinical trials on immuno-oncology combination therapies in a wide variety of indications with more than 4,600 subjects enrolled in China, the U.S., Turkey, Poland, Georgia and other countries and regions. From which, HANSIZHUANG in combination with chemotherapy for the first-line treatment of patients with unresectable locally advanced/recurrent or metastatic ESCC has been approved by the China National Medical Products Administration (NMPA) in September 2023, providing a new treatment option for patients with ESCC.
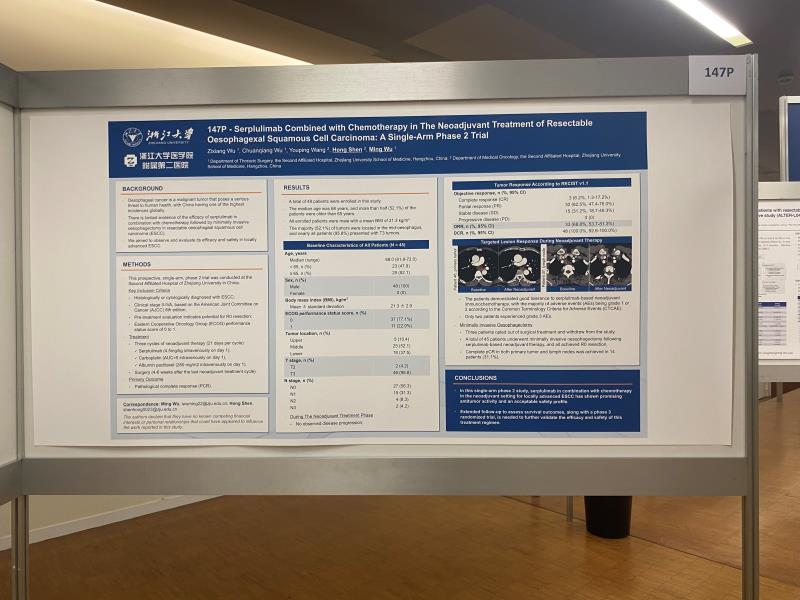
Title: Serplulimab Combined with Chemotherapy in The Neoadjuvant Treatment of Resectable Oesophagexal Squamous Cell Carcinoma: A Single-Arm Phase 2 Trial
Time: December 12, 2024(CET)
Study Design:
Key Inclusion Criteria:
-Histologically or cytologically diagnosed with ESCC;
-Clinical stage II-IVA, based on the American Joint Committee on Cancer (AJCC) 8th edition;
-Pre-treatment evaluation indicates potential for R0 resection;
-Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 1.
Treatment:
Three cycles of neoadjuvant therapy (21 days per cycle):Serplulimab (4.5mg/kg intravenously on day 1), Carboplatin (AUC=5 intravenously on day 1),Albumin paclitaxel (260 mg/m2 intravenously on day 1).Surgery (4-6 weeks after the last neoadjuvant treatment cycle).
Primary Outcome:
Pathological complete response (PCR).
Results:
Three patients opted out of surgical treatment and withdrew from the study. A total of 45 patients underwent minimally invasive oesophagectomy following serplulimab-based neoadjuvant therapy, and all achieved R0 resection. Complete pCR in both primary tumor and lymph nodes was achieved in 14 patients (31.1%). Only two patients experienced grade 3 AEs.
Conclusion:
In this single-arm phase 2 study, serplulimab in combination with chemotherapy in the neoadjuvant setting for locally advanced ESCC has shown promising antitumor activity and an acceptable safety profile.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases and ophthalmic diseases. Up to date, 6 products have been launched in China, 3 have been approved for marketing in overseas markets, 25 indications are approved worldwide, and 4 marketing applications have been accepted for review in China, the U.S. and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centre and Shanghai-based commercial manufacturing facilities certificated by China, the EU and U.S. GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering over 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as the backbone. Apart from the launched products HANLIKANG (rituximab), the first China-developed biosimilar, HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANDAYUAN (adalimumab) and HANBEITAI (bevacizumab), the innovative product HANSIZHUANG has been approved by the NMPA for the treatment of MSI-H solid tumors, squamous non-small cell lung cancer (sqNSCLC), extensive-stage small cell lung cancer (ES-SCLC), esophageal squamous cell carcinoma (ESCC) and non-small cell lung cancer (nsNSCLC), making it the world’s first anti-PD-1 mAb for the first-line treatment of SCLC. What’s more, Henlius has conducted over 30 clinical studies for 16 products, expanding its presence in major markets as well as emerging markets.
