Shanghai, China, June 28, 2024 - Shanghai Henlius Biotech, Inc. (2696.HK) announced that a phase 1 clinical trial (NCT05679258) of the company’s daratumumab biosimilar HLX15, a recombinant anti-CD38 fully human monoclonal antibody injection, met its primary endpoint.
HLX15 is a fully human anti-CD38 IgG1κ monoclonal antibody independently developed by Henlius. The indication to be developed for HLX15 is multiple myeloma (MM). In accordance with the biosimilar guidelines of NMPA, EMA, and FDA, HLX15 has been developed strictly following the principles of stepwise development, comparability and similarity assessment and has been compared head-to-head with reference daratumumab via analytical studies and preclinical studies. The results of these preclinical studies showed that HLX15 is highly similar to reference daratumumab. And the results of this Phase 1 study suggest that HLX15 had similar pharmacokinetic characteristics, as well as comparable safety and immunogenicity profiles to the US-, EU-, and CN-sourced daratumumab ("reference drug").

MM is a clonal plasma cell dysplasia, one of the top hematological malignancies in many countries which mainly develops in elderly population [1]. According to GLOBOCAN 2022, the 5-year prevalence for MM was over 540,000 and there were around 188,000 new cases and over 121,000 deaths have been reported worldwide in 2022. In China, there are more than 85,000 patients in 5 years as of 2022, more than 30,000 new patients and more than 18,000 deaths have been reported [2,3].
CD38, also known as cyclic adenosine diphosphate (ADP) ribose hydrolase, is usually expressed in plasma cells, lymphoid cells, and myeloid cells. High expression of CD38 is present in varies of hematologic malignancies, especially at all stages along disease progression of MM. Considering the expression profile of CD38, it is thought to be a cell surface marker of hematological malignancies and an ideal target for the treatment of multiple myeloma [4,5].
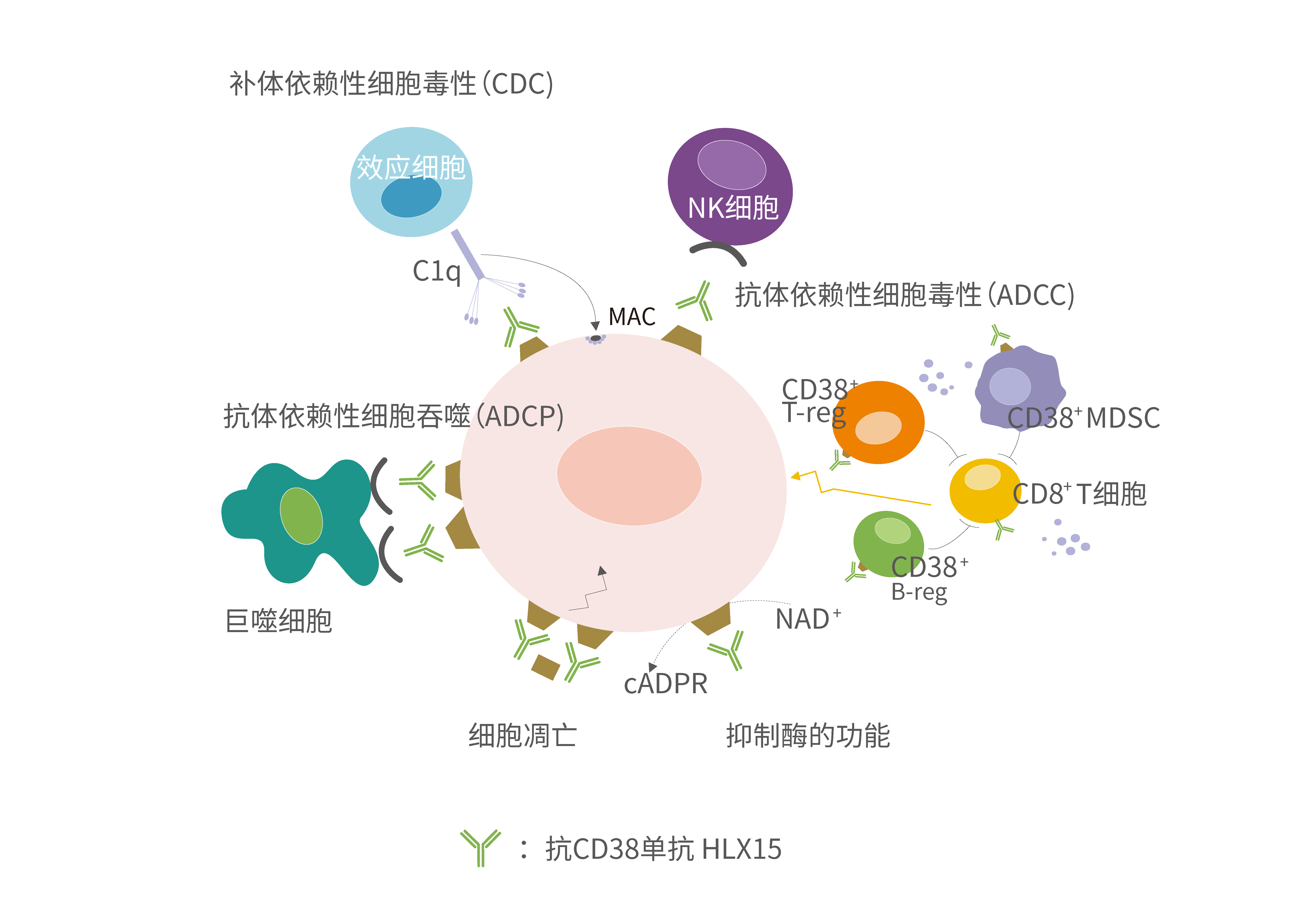
On one hand, HLX15 can directly bind to CD38 expressed on the surface of tumour cells, inducing tumour cell lysis and apoptosis through complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), and several other pathways such as Fc mediated cross linking to achieve a quick response of tumour cells. In addition, HLX15 can also reduce MM cells by reducing myeloid-derived suppressor cells and depleting CD38-positive immunomodulatory T and B cells.
Henlius will always place a high value on patients’ needs and clinical data and will continue to diversify innovation by strengthening internal innovation capabilities and external collaboration, extending new drug forms, and processing more clinical trials, to benefit patients around the world.
【Reference】
[1] Chinese Hematology Association; Chinese Society of Hematology. Zhonghua Nei Ke Za Zhi. 2022;61(5):480-487. doi:10.3760/cma.j.cn112138-20220309-00165
[2] WHO-Multiple myeloma - Global Cancer Observatory-2022.
[3] Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and Management of Multiple Myeloma: A Review. JAMA. 2022;327(5):464-477.
[4] Bonello, F., M. D'Agostino, M. Moscvin, C. Cerrato, M. Boccadoro, and F. Gay. 2018. 'CD38 as an immunotherapeutic target in multiple myeloma', Expert Opin Biol Ther, 18: 1209-21.
[5] Morandi, F., A. L. Horenstein, F. Costa, N. Giuliani, V. Pistoia, and F. Malavasi. 2018. 'CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma', Front Immunol, 9: 2722.
