Recently, Henlius has made a breakthrough in asymmetric multichain bispecific antibody cell line development (CLD), in which the stable monoclonal cell line increased the titer to 6g/L, and over 90% of clones expressed the desired products, such results have exceeded the industry's average level. Based on the achievement, the company was invited to the BioProcess International US West Conference. Yanling Wang, CLD/PEX Director of Henlius US Innovation Center, gave an oral presentation on behalf of the team and participated in the panel discussion on "Improving Yield and Speed for Novel Modality Production".
With the advancement in biotechnology, innovative drug format has evolved from natural monospecific antibody and fusion protein to multifunctional and multi-specific modality like bispecific antibody. Therefore, the industry has higher requirement for antibody expression level and cell line development quality. However, the traditional random integration (RI) of the gene of interest into the host genome makes the cell line screening labor-intensive and time-consuming. The asymmetric bispecific antibody preparation process is prone to mispairing of light chains and heavy chains, and high level of product-related impurities that are hard to separate from the targeted product. Discussion is increasingly turning to the bispecific antibody technology and site-specific integration (SSI) based on transposon enzymes. The multiple site-specific integrations of SSI and the related stable cell pools/single clones show the advantages in stability and consistency across batches that improve the efficiency of the drug development.
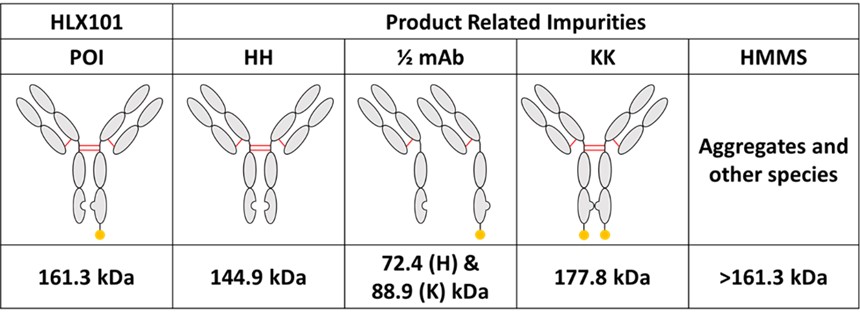
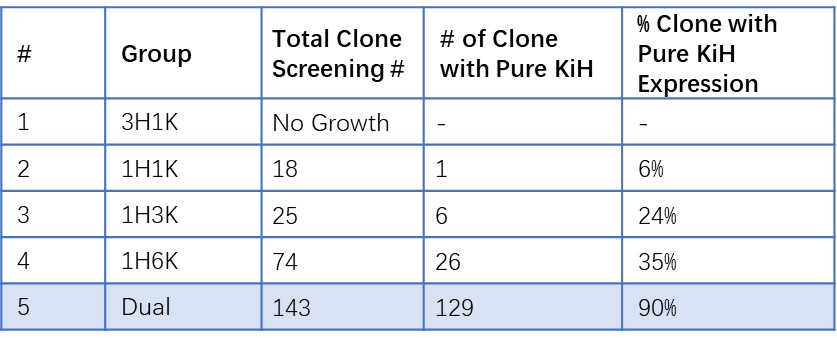
Henlius makes full use of innovative technologies and methods to develop innovative drugs. HLX101 is an asymmetric fusion antibody, developed by Henlius independently, composed of two different heavy chains and one common light chain (LC) Knobs-into-holes (KiH) mutations were designed in the CH3 region. The C-terminal of heavy chain-knob has a cytokine attached (Knob-Cytokine) and the heavy chain-hole does not. The team of Henlius adopted a series of innovative technologies, by exploring vector configuration and transfection ratio, then utilized SSI transposon system and high-throughput clone screening method to construct the stable pools of HLX101. The stable pools increased to a higher titer of >6g/L and were rich in clones (increased from 6% to 90%) with the desired product quality attributes and fewer product-related impurities. Besides, the performance of single clones is consistent with stable pool source. Herein, an inspired CLD strategy was developed for asymmetric multichain bispecific antibodies, improved the overall screening efficiency and candidate clone quality, and laid a firm foundation for innovative product development.
Henlius keeps the momentum for a diversified innovation by enhancing internal innovation capacities and external collaboration with more strategic partners. The two global innovation centers, located in China and the U.S., are respectively responsible for early-stage development, late-stage development, and process development and application. Henlius aggressively pushes its early R&D research forward by looking into novel coupling techniques in effort to grow its presence in antibodies with independent development accounts for over 80% of the product pipeline. Meanwhile, Henlius expands its in-licensing and collaboration on external innovative assets through collaboration with strategic partners to meet global clinical needs.
