The 24th Annual Meeting of Chinese Society of Clinical Oncology (CSCO) is held online and in-person from 25th to 29th September 2021. In this meeting, a phase 2 study of serplulimab (HLX10), an innovative anti-PD-1 mAb independently developed by Henlius, in patients with advanced hepatocellular carcinoma (HCC) clinical study has been selected for the Innovation Session due to its excellent results. In April, the New Drug Application (NDA) of serplulimab for the treatment of MSI-H solid tumours was accepted by the National Medical Products Administration (NMPA) and granted priority review, which is expected to be approved in the first half of 2022. What's more, the NDA of serplulimab for the treatment of squamous non-small cell lung cancer is also under review.
With the "Combo+Global" strategy, serplulimab has been approved for clinical trials in China, the United States, the European Union, as well as other countries and regions. To evaluate the safety and efficacy of serplulimab, Henlius has conducted 10 immuno-oncology therapy clinical studies covering cancers with high incidence rates, including lung cancer, esophageal cancer, hepatocellular cancer, gastric cancer, head and neck cancer, etc. Up to date, about 2300 patients have been enrolled worldwide, proving that the quality of serplulimab has built trust in foreign markets.
Details of this study are as follows:
Title: Phase 2 study of HLX10 (a novel anti-PD-1 antibody) plus HLX04 (an anti-VEGF antibody) in patients with advanced hepatocellular carcinoma (ID: 9949)
Leading PI: Jia Fan, Zhongshan Hospital, Fudan University; Zhenggang Ren, Zhongshan Hospital, Fudan University
Form: Oral presentation
Presenter: Zhenggang Ren, Zhongshan Hospital, Fudan University
Time: 2021.09.28 15:00–15:10,Main Session,Innovation session 2 (Shanghai)
Study design
This open-label, multi-centre phase 2 study aimed to evaluate the efficacy, safety and tolerability of HLX10 in combination with HLX04 for patients with advanced hepatocellular carcinoma. Eligible patients were recruited to four treatment groups. Patients with progressive disease or intolerable toxicities after standard therapy were enrolled to group A, B and C and patients with no prior systemic therapy were enrolled to group D to receive intravenous infusion (every two weeks) of HLX10 3 mg/kg plus HLX04 5 mg/kg, HLX10 3 mg/kg plus HLX04 10 mg/kg, HLX10 3 mg/kg, HLX10 3 mg/kg plus HLX04 10 mg/kg, respectively. The endpoints included objective response rate (ORR) assessed by independent radiological review committee (IRRC) and investigators per RECIST v1.1, overall survival (OS), progression-free survival (PFS), duration of response (DoR), safety, etc.
Results
Efficacy
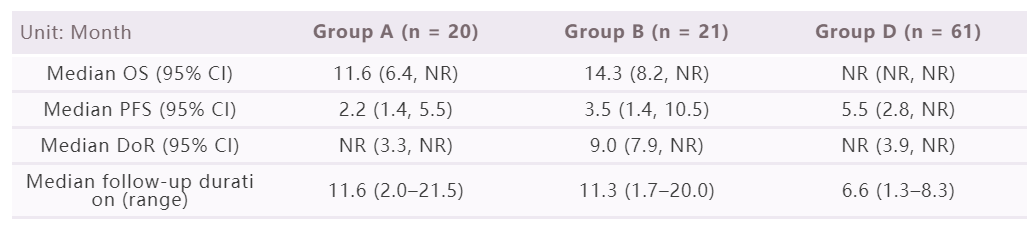
By cut-off date of 4 August 2021, since the median follow-up duration of group C is too short, here we mainly report the results collected from group A (n = 20), B (n = 21) and D (n = 61). The IRRC assessed ORR of group A, B and D were 30.0% (95% CI: 11.9, 54.3), 14.3% (95% CI: 3.0, 36.3) and 26.2% (95% CI: 15.8, 39.1), respectively. The other efficacy outcomes are as follows.
Safety
The results demonstrated that HLX10 plus HLX04 was safe and well-tolerated.
Conclusion
The results demonstrated that HLX10 in combination with HLX04 had encouraging antitumour activity with a manageable safety profile in patients with advanced hepatocellular carcinoma. HLX10 plus HLX04 possessed the potential to provide an alternative treatment option for suitable patients.
