On February 3, 2023 (UTC +8), the results of ASTRUM-007, Henlius’ phase 3 clinical study of anti-PD-1 mAb HANSIZHUANG (serplulimab) plus chemotherapy as first-line treatment for patients with PD-L1 combined positive score ≥1 esophageal squamous cell carcinoma (ESCC), were published in the international leading journal Nature Medicine (impact factor 87.241). The leading principal investigator of ASTRUM-007 was Professor Jing Huang from National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.

In November and December 2022, the results of ASTRUM-007 have been released as oral presentations at the Annual Meeting of Chinese Society of Clinical Oncology (CSCO) and the European Society for Medical Oncology (ESMO) Asia Congress, respectively. Published in Nature Medicine, ASTRUM-007 once again demonstrated the innovation power as well as the research and development strength of Chinese pharmaceutical enterprises in anti-PD-1 mAb development on the international academic stage. Based on the results of ASTRUM-007, the new drug application (NDA) of serplulimab for the treatment of ESCC has been accepted by the National Medical Products Administration (NMPA). Recently, HANSIZHUANG was recommended for the first-line treatment of esophageal cancer by the 2022 Chinese Guidelines for Radiotherapy of Esophageal Cancer.
Prof. Jing Huang, the corresponding author, as well as the leading principal investigator of the study, said, “Serplulimab plus chemotherapy significantly improved PFS and OS in patients with previously untreated, PD-L1–positive, advanced ESCC, with a manageable safety profile. I would like to thank all patients, their families, as well as study investigators who contributed to this study. We are hoping that the approval of HANSIZHUANG for the treatment of ESCC comes soon and bring a new treatment option to patients with ESCC.”
Mr. Jason Zhu, President of Henlius, said, "It's the second time that HANSIZHUANG has been published in an international leading journal after JAMA, which not only confirmed the excellent quality of HANSIZHUANG but also showed the company's international clinical study capacity and innovation strength. We have achieved an overall layout in lung cancer and gastrointestinal cancer, and we are striving diligently to get more indications of HANSIZHUANG on the market. HANSIZHUANG is expected to help more patients around the world with high-quality, Chinese-developed biologics."
A new option for the first-line immuno-oncology therapy of ESCC
Esophageal cancer is one of the most common malignancies worldwide, mainly classified as squamous cell carcinoma and adenocarcinoma, and ESCC accounts for more than 84% of esophageal cancer[1]. Esophageal cancer is highly prevalent in China. According to the estimates of cancer incidence and mortality in China in 2016, there were 252,500 new cases and 193,900 deaths related to esophageal cancer, ranking sixth and fifth in all malignant tumours in China, respectively[2]. As the symptoms of early esophageal cancer are often subtle, most patients are diagnosed at mid- to late-stage, missing out on surgical treatment. The main treatment for advanced patients is systematic treatment (chemotherapy or targeted therapy), but the treatment efficacy is often limited, with high recurrence and metastasis rate. Therefore, new drugs and treatments are urgently needed. In recent years, immuno-oncology therapy has become one of the research priorities at home and abroad. Many studies have shown that anti-PD-1 mAb combined with chemotherapy can bring survival benefits to patients with esophageal cancer. Immune checkpoint inhibitor combined with chemotherapy has become the standard first-line treatment for advanced esophageal cancer in China[3].
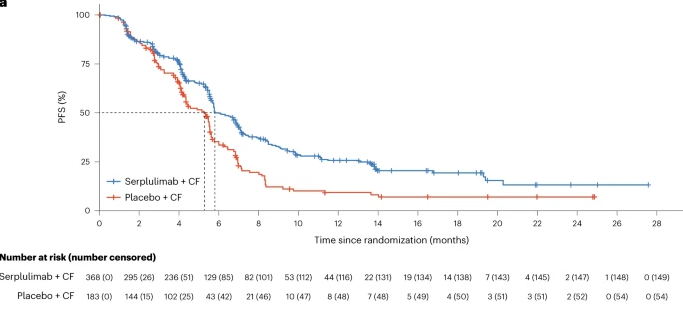
ASTRUM-007 (NCT03958890) is a randomised, double-blind, multicentre, phase 3 study aiming to compare the efficacy and safety of serplulimab versus placebo in combination with chemotherapy in patients with previously untreated, PD-L1–positive (combined positive score ≥1), advanced ESCC. 551 eligible patients were randomised 2:1 to receive serplulimab or placebo combined with 5-fluorouracil and cisplatin intravenously every 2 weeks. Median PFS was significantly longer in serplulimab plus chemotherapy group than that in placebo plus chemotherapy group (5.8 vs. 5.3 months; hazard ratio [HR] 0.60, 95% confidence interval [CI] 0.48–0.75; p<0.0001). Median OS was also significantly improved with the addition of serplulimab (15.3 vs. 11.8 months; HR 0.68, 95% CI 0.53–0.87; p=0.0020). The safety profile of serplulimab plus chemotherapy was manageable. In conclusion, serplulimab in combination with chemotherapy administered every 2 weeks significantly improved PFS and OS compared with chemotherapy alone in first-line treatment of PD-L1–positive advanced ESCC, which can be considered as a new standard option for this patient population.
HANSIZHUANG clinical layout covered high incidence tumours, including lung cancer and gastrointestinal cancer
HANSIZHUANG (serplulimab) is the first innovative mAb developed by Henlius. Since launched in March 2022, HANSIZHUANG has been approved by the NMPA for the treatment of MSI-H solid tumours, squamous non-small cell lung cancer and extensive-stage small cell lung cancer (ES-SCLC), benefiting more than 15,000 Chinese patients. Henlius actively promotes HANSIZHUANG in conjunction with in-house products of the company such as tumour-specific target, angiogenesis target, immunotherapeutic target, etc. and chemotherapy drugs to conduct immune combination therapies. Underpinned by the patient-centric strategy, Henlius has carried out a differentiated and multi-dimensional layout in the field of gastrointestinal cancer and lung cancer, covering a wide variety of indications, such as lung cancer, hepatocellular carcinoma, ESCC, head and neck squamous cell carcinoma and gastric cancer, etc. To date, more than 3,100 subjects have been enrolled worldwide for HANSIZHUANG clinical trials.
In the field of gastrointestinal cancer, HANSIZHUANG has been approved for the treatment of MSI-H solid tumours, which could benefit patients with MSI-H colorectal cancer and MSI-H gastric cancer. In addition, PD-1 inhibitors are less explored in neoadjuvant/adjuvant therapies for gastric cancer, and Henlius has led the way with a phase 3 clinical study, striving to benefit gastric cancer patients from the early line of immunotherapy. As for the first-line lung cancer treatment, HANSIZHUNAG is recognized by international prestigious academic journals after the publication of ASTRUM-005, an ES-SCLC phase 3 study, in JAMA, one of the top four medical journals, in September 2022. Moreover, HANSIZHUANG has been granted orphan-drug designations for the treatment of SCLC by the United States Food and Drug Administration (FDA) and the European Commission (EC), respectively. Furthermore, HANSIZHUANG was recommended by the 2022 CSCO Guidelines for Diagnosis and Treatment of SCLC for the treatment of ES-SCLC.
Looking forward, Henlius will actively improve efficiency through innovations, focusing on unmet medical needs to bring more high-quality and affordable therapies to patients worldwide.
About Nature Medicine
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine on the basis of its originality, timeliness, interdisciplinary interest and impact on improving human health. Nature Medicine also publishes commissioned content, including News, Reviews and Perspectives, aimed at contextualizing the latest advances in translational and clinical research to reach a wide audience of M.D. and Ph.D. readers. All editorial decisions are made by a team of full-time professional editors.
About HANSIZHUANG
HANSIZHUANG (recombinant humanized anti-PD-1 monoclonal antibody injection, generic name: serplulimab injection) is the first innovative monoclonal antibody developed by Henlius and and the world’s first anti-PD-1 mAb for the first-line treatment of SCLC. Up to date, 3 indications are approved for marketing in China, 1 NDA has been accepted by the NMPA, and more than 10 clinical trials are ongoing across the world.
HANSIZHUANG was launched in March 2022 and has been approved by the NMPA for the treatment of MSI-H solid tumours, squamous non-small cell lung cancer (sqNSCLC) and extensive-stage small cell lung cancer (ES-SCLC). Its synergy with in-house products of the company and innovative therapies are being actively promoted. It has successively obtained clinical trial licenses in China, the United States, the European Union and other countries and regions to initiate 12 clinical trials on immuno-oncology combination therapies in a wide variety of indications, such as lung cancer, esophageal carcinoma, head and neck squamous cell carcinoma and gastric cancer, etc., and covering the full range of first-line treatments of lung cancers. As of now, the company has enrolled more than 3,100 subjects in China, Turkey, Poland, Georgia and other countries and regions, and the proportion of White is over 30% in two MRCTs, making HANSIZHUANG an anti-PD-1 mAb with one of the largest global clinical data pools. The NDA of the first-line treatment for esophageal squamous cell carcinoma (ESCC) has been accepted by the NMPA. Furthermore, HANSIZHUANG was respectively recommended for the first-line treatment of ES-SCLC in the 2022 CSCO Guidelines for Diagnosis and Treatment of Small Cell Lung Cancer (SCLC) and the trentment of esophageal cancer in the 2022 Chinese Guidelines for Radiotherapy of Esophageal Cancer. The associated clinical trial became the first study published in JAMA on SCLC immunotherapy, and the clinical study of ESCC was published in Nature Medicine. Furthermore, serplulimab was also granted orphan drug designations by the FDA and EC for the treatment of SCLC, and the first patient has been dosed in a bridging head-to-head trial in the United States to comparing HANSIZHUANG to standard of care atezolizumab (anti-PD-L1 mAb) for the first-line treatment of ES-SCLC.
References
[1] Arnold, M., Ferlay, J., van Berge Henegouwen, M.I. & Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69, 1564-1571 (2020).
[2] Zheng, R., Zhang, S., Zeng, H., et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center 2, 1-9 (2022).
[3] 食管癌诊疗指南(2022年版). 中国国家卫生健康委员会.
*图片素材来源于Nature Medicine官网
