Recently, the latest clinical data of two Henlius products were released online and will be presented in poster sessions at the 2024 ASCO Gastrointestinal Cancers Symposium (ASCO GI), namely, the phase 2/3 study (HLX10-015-CRC301) of Henlius’ NMPA approved anti-PD-1 monoclonal antibody (mAb) HANSIZHUANG (serplulimab) in metastatic colorectal cancer (mCRC) with Professor Rui-Hua Xu of Sun Yat-Sen University Cancer Center as the leading principal investigator, and the phase 2 study (HLX22-GC-201) of Henlius’ novel anti-HER2 mAb, HLX22, combined with HANQUYOU (trastuzumab for injection, HLX02, trade name in Europe: Zercepac®; trade names in Australia: Tuzucip® and Trastucip®) and chemotherapy for the first-line treatment of HER2-positive gastric/gastroesophageal junction (G/GEJ) cancer with Professor Jin Li of Shanghai East Hospital, School of Medicine, Tongji University as the leading principal investigator of this study.
HLX10-015-CRC301
Colorectal cancer (CRC) is one of the most common malignancies globally. Over 1.9 million newly diagnosed cases and more than 900,000 deaths were estimated in 2020[1]. The standard of care for metastatic CRC (mCRC) involves the combination of vascular endothelial growth factor (VEGF) inhibitor, such as bevacizumab, and systemic chemotherapy[2-4]. Previously, HANSIZHUANG was approved in China for the treatment of MSI-H solid tumours in March 2022 based on its excellent efficacy data, and then was recommended by the CSCO Guidelines for Colorectal Cancer and the CSCO Clinical Practice Guidelines on Immune Checkpoint Inhibitor. The company is continuing to explore immuno-oncology therapy for this arena, with the goal of delivering more effective treatment for a broader population of patients. The results of this study demonstrated that serplulimab plus HLX04 (a bevacizumab biosimilar) and XELOX markedly prolonged PFS compared to placebo plus bevacizumab and XELOX, with a manageable safety profile.
HLX22-GC-201
Gastric/gastroesophageal junction (G/GEJ) cancer represents a global healthcare challenge with more than 1 million new cases estimated in 2020[1]. G/GEJ cancer is often diagnosed at the advanced stage, and the prognosis of advanced G/GEJ cancer is poor, with a 5-year relative survival rate of only 6%[5,6]. And the prognosis for patients with HER2-positive disease used to be even worse than those with HER2-negative disease[5-7]. HLX22 is an innovative anti-HER2 mAb that was introduced from AbClon, Inc. and further researched and developed by Henlius. HLX22 can bind to HER2 subdomain IV at a different binding site from trastuzumab, which allows the simultaneous binding of HLX22 and trastuzumab to HER2. The pre-clinical studies showed that the combination therapy of HLX22 and trastuzumab would inhibit the cell proliferation induced by epidermal growth factor (EGF) and Histidine-Rich Glycoprotein 1 (HRG1) and enhance the antitumor activity in vitro and in vivo. The phase 1 clinical trial of HLX22 demonstrates that HLX22 is well tolerated and has good safety profiles. As of now, no similar dual HER2 blockade therapy for the treatment of HER2-positive gastric cancer has received approval for commercialization globally.
The data released at ASCO GI 2024 are as follows:
Title
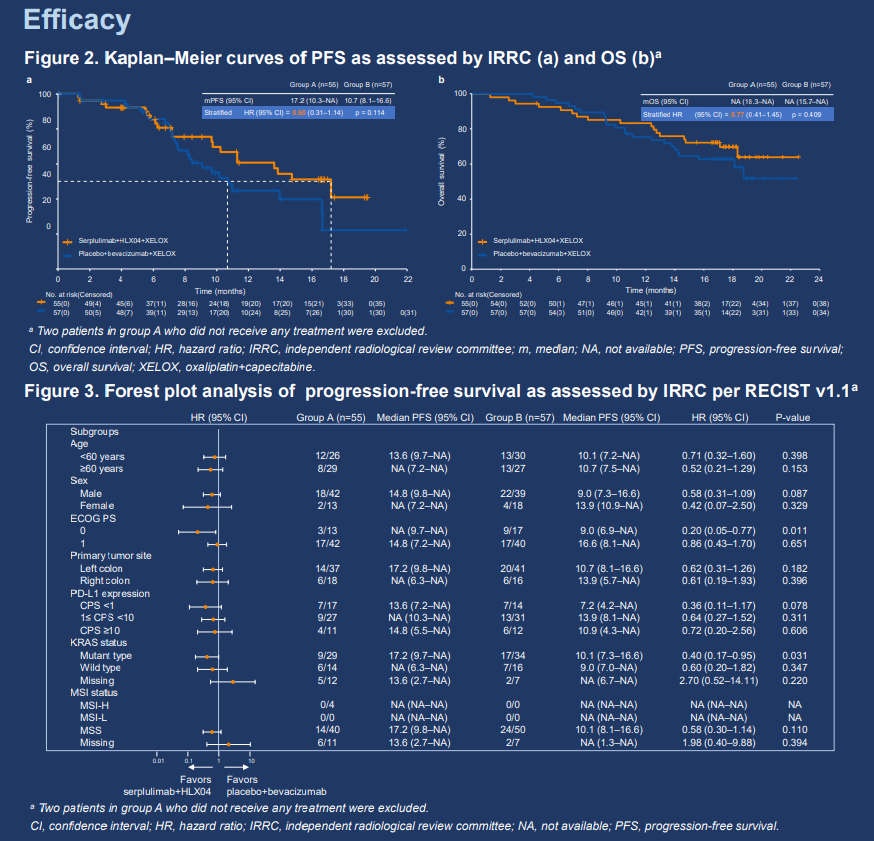
First-line serplulimab plus HLX04 and XELOX versus placebo plus bevacizumab and XELOX in metastatic colorectal cancer: a phase 2/3 study
Study design
This was a randomized, double-blind, multicenter phase 2/3 study; current report will focus on the phase 2 part. Patients with unresectable metastatic/recurrent colorectal adenocarcinoma and no prior systemic therapy were randomized 1:1 to receive serplulimab (300 mg, Q3W IV) plus HLX04 (a bevacizumab biosimilar, 7.5 mg/kg, Q3W IV) and XELOX (group A) or placebo plus bevacizumab and XELOX (group B). Randomization was stratified by PD-L1 expression level (CPS <1 vs. 1≤ CPS <50 vs. CPS ≥50), ECOG PS score (0 vs. 1), and primary tumor site (left- vs. right-sided). The primary endpoint was IRRC-assessed PFS per RECIST 1.1. Secondary endpoints included other efficacy endpoints, safety, pharmacokinetics, biomarker explorations, and quality-of-life assessments.
Results
Between July 16, 2021 and January 20, 2022, 114 patients were enrolled and randomly assigned to group A (n = 57) and group B (n = 57). 83 (72.8%) patients were male. All patients had stage IV colorectal cancer (CRC), and 90 out of the 94 (95.7%) with available MSI status were MSS. Current report will focus on efficacy and safety findings in the modified intention-to-treat population, which included only patients who received study treatment (group A, n = 55; group B, n = 57). As of June 1, 2023 (data cutoff), median follow-up duration was 17.7 months. IRRC-assessed median PFS was longer in group A than in group B (17.2 vs. 10.7 months; stratified HR 0.60, 95% CI 0.31–1.14). Median OS was not reached in either group (stratified HR 0.77, 95% CI 0.41–1.45). 36 (65.5%) patients in group A and 32 (56.1%) in group B had grade ≥3 treatment-related adverse events (AEs), most commonly neutrophil count decreased (21.8% vs. 10.5%) and platelet count decreased (16.4% vs. 10.5%). Grade ≥3 immune-related AEs were reported for 5 (9.1%) patients in group A and 1 (1.8%) in group B. Treatment-related deaths occurred in 4 (7.3%) patients in group A and 3 (5.3%) in group B.
Conclusion
Serplulimab plus HLX04 and XELOX prolonged PFS compared to placebo plus bevacizumab and XELOX. Improvements in other efficacy endpoints were also observed with a manageable safety profile in patients with metastatic CRC. Serplulimab plus HLX04 and XELOX is a promising first-line treatment option for metastatic CRC patients that warrants further investigation.
____________________________________________________________________
Title
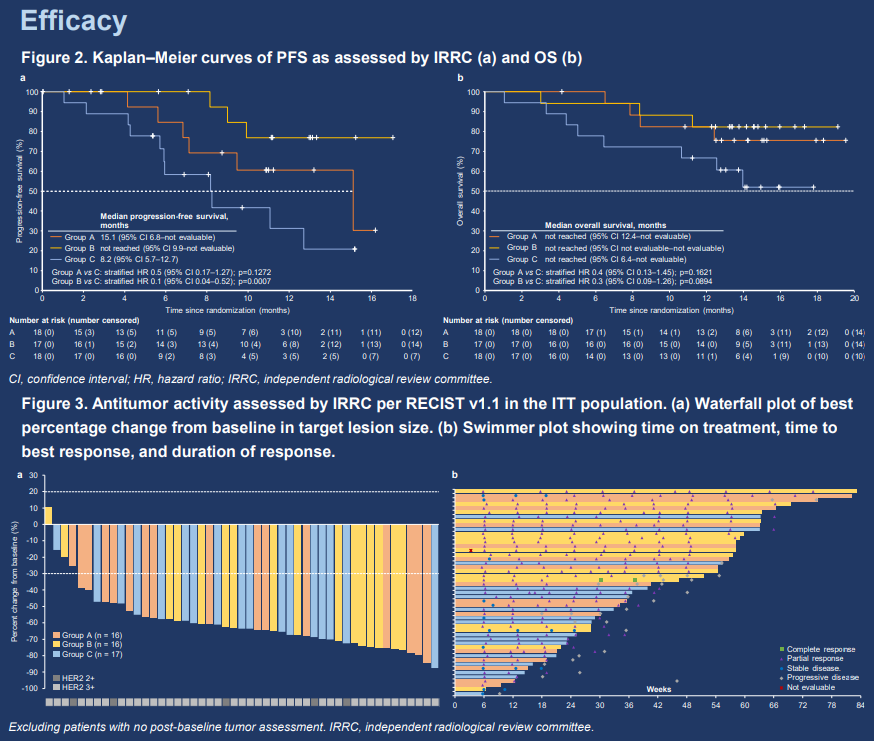
HLX22 plus HLX02 and XELOX for first-line treatment of HER2-positive locally advanced or metastatic gastric/gastroesophageal junction cancer: a randomized, double-blind, multicenter phase 2 study
Study design
The study was unblinded 3 months after the last patient was enrolled. Patients with locally advanced or metastatic HER2-positive gastric/gastroesophageal junction (G/GEJ) cancer and had not received prior systemic antitumor therapy were enrolled. The study consisted of 2 parts; current report will focus on part 1. In part 1, patients were randomized 1:1:1 to receive HLX22 (a novel anti-HER2 monoclonal antibody) 25 mg/kg + HLX02 (a trastuzumab biosimilar) + XELOX (group A), HLX22 15 mg/kg + HLX02 + XELOX (group B), or placebo + HLX02 + XELOX (group C) in 3-week cycles. Primary endpoints were PFS and ORR assessed by IRRC per RECIST v1.1. Secondary endpoints included other efficacy measures and safety.
Results
As of July 30, 2023 (data cutoff), 53 patients were randomized to group A (n = 18), B (n = 17), and C (n = 18), and were followed up for a median of 14.3 months. 44 (83.0%) patients were male. Main efficacy results are presented in Table 1. Tumor assessments reported in Table 1 were performed by IRRC. Treatment-related adverse events (TRAEs) occurred in 18 (100.0%), 16 (94.1%), and 17 (94.4%) patients in the respective groups. Serious TRAEs were observed in 5 (27.8%) patients in group A, 1 (5.9%) in group B, and 1 (5.6%) in group C. Only 1 (5.6%) patient in group C had a grade 5 TRAE.
Conclusion
Adding HLX22 to HLX02 + XELOX improved survival and antitumor response in patients with HER2-positive G/GEJ cancer in the first-line setting, with a manageable safety profile.
Table 1. Efficacy results

NE, not evaluable; NR, not reached.
【References】
[1] Sung H. et al. CA Cancer J Clin 2021;71(3):209-49.
[2] Iwasa, S. et al. Cancer Commun (Lond) 43, 519-522 (2023).
[3] Hurwitz, H. et al. N Engl J Med 350, 2335-2342 (2004).
[4] Benson, A. B. et al. J Natl Compr Canc Netw 20, 1139-1167 (2022).
[5] Ajani JA. et al. J Natl Compr Canc Netw 2022;20(2):167-92.
[6] Alsina M. et al. Nat Rev Gastroenterol Hepatol 2023;20(3):155-70.
[7] Gravalos C. et al. Ann Oncol 2008;19(9):1523-9.
