Shanghai, China, December 2, 2022 - Shanghai Henlius Biotech, Inc. (2696.HK) announced that the first subject was dosed for a phase 1 clinical trial of HLX60 (recombinant anti-GARP humanised monoclonal antibody injection), independently developed by the company, for the treatment of solid tumours and lymphomas. HLX60 is the first investigational new drug (IND) approved anti-GARP monoclonal antibody (mAb) in China and is expected to be the first-in-class anti-GARP mAb.
The glycoprotein-A repetitions predominant (GARP) is highly expressed on the surface of activated Tregs and platelets and acts as a docking receptor by binding to latent transforming growth factor-β1 (LTGF-β1) [1]. Transforming growth factor-β (TGF-β) is a pleiotropic cytokine expressed by the majority of cells and found in many tissues. There are three TGF-β receptor ligands: TGF-β1, TGF-β2, and TGF-β3, with TGF-β1 playing critical roles in a variety of biological processes including cell proliferation, development, apoptosis, fibrosis, angiogenesis, wound healing, cancer, and many others [2–4]. It concentrates LTGF-β1 on the cell surface and releases active TGF-β1 in the tumour microenvironment (TME), thus inhibiting anti-tumour immune response and inducing tumour cell growth, proliferation, and invasion [5-6].
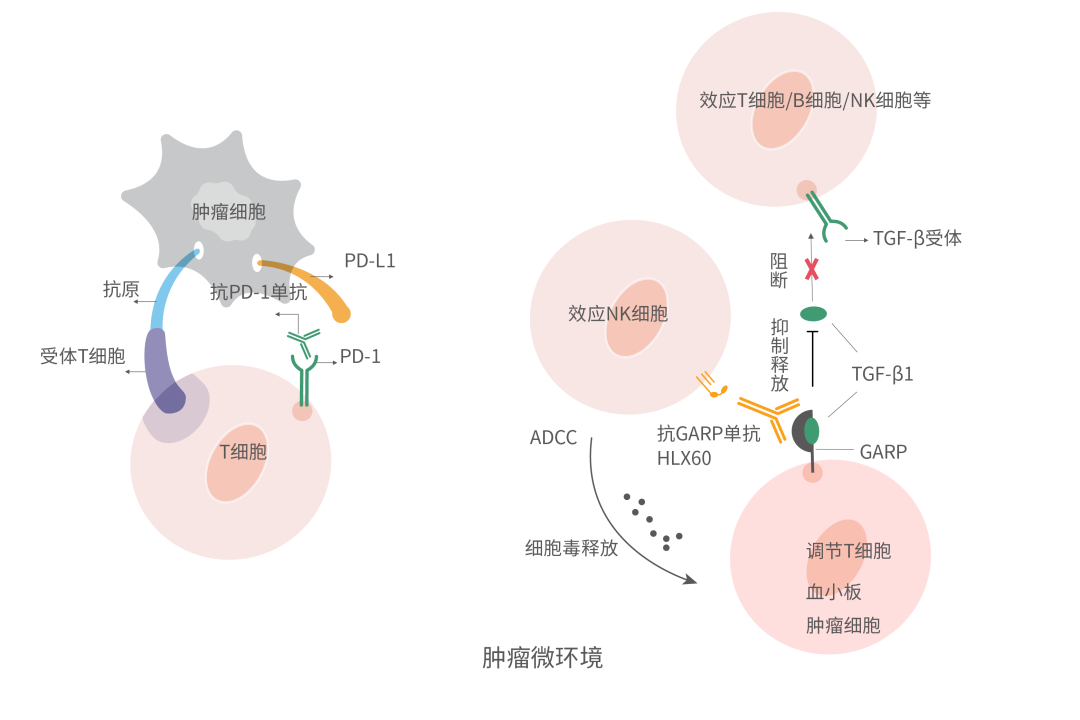
HLX60 is a self-developed novel anti-GARP mAb that binds to GARP and specifically blocks the release of GARP mediated TGF-β1, thus relieving the immunosuppression caused by TGF-β1, reversing the immunosuppressive TME, and improving anti-tumour immune response. Furthermore, by inducing antibody dependent cell-mediated cytotoxicity (ADCC) effect, HLX60 can deplete GARP positive tumour cells as well as immunosuppressive cells such as Tregs, thus enhancing anti-tumour effect. Several pre-clinical studies have shown that HLX60 has anti-tumour effects and a good safety profile. Plus, HLX60 in combination with HANSIZHUANG (serplulimab), an innovative anti-PD-1 mAb independently developed by Henlius, demonstrates a synergistic effect in inhibiting tumour growth. Recently, the phase 1 clinical trial application of HLX60 in combination with HANSIZHUANG for the treatment of advanced or metastatic solid tumours has been approved in Australia.
Underpinned by the patient-centric strategy, Henlius has built an innovative product pipeline with many emerging targets, including PD-1/L1, LAG-3, GARP, TIGIT, BRAF, etc. Regarding antibody technology as a core, Henlius will continue conducting clinical studies for more innovative products in bispecific antibodies and antibody-drug conjugates (ADC) and exploring combination therapies with improved efficacy to provide patients with quality and affordable biologics.
About NCT05606380
This open-label, dose-escalation, first-in-human phase 1 study aims to evaluate the safety and tolerability of HLX60 in patients with advanced/metastatic solid tumours or lymphomas. This study follows an accelerated titration design (ATD) in combination with “3+3” design. Eligible patients will be given different doses of intravenous HLX60 (Q3W: 0.5, 2, 5, 15, and 25 mg/kg) as monotherapy. The primary endpoints of this study are the dose-limiting toxicities (DLT) and the maximum tolerated dose (MTD) assessed within 3 weeks after the first dose of HLX60. Secondary endpoints include safety, pharmacokinetic parameters, pharmacodynamic characteristics, immunogenicity, efficacy, and the exploration of biomarkers.
Reference
[1] Roubin R, Pizette S, Ollendorff V, Planche J, Birnbaum D and Delapeyriere O. Structure and developmental expression of mouse Garp, a gene encoding a new leucine rich repeat-containing protein. The International journal of developmental biology. 1996; 40(3):545-555.
